Copper(I) oxide
| Copper(I) oxide | |
|---|---|
|
Copper(I) oxide |
|
|
Other names
Cuprous oxide |
|
| Identifiers | |
| CAS number | 1317-39-1 |
| PubChem | 10313194 |
| ChemSpider | 8488659 |
| UNII | T8BEA5064F |
| EC number | 215-270-7 |
| KEGG | C18714 |
| RTECS number | GL8050000 |
| Jmol-3D images | Image 1 Image 2 |
|
|
|
|
| Properties | |
| Molecular formula | Cu2O |
| Molar mass | 143.09 g/mol |
| Appearance | brownish-red solid |
| Density | 6.0 g/cm3 |
| Melting point |
1235 °C, 1508 K, 2255 °F |
| Boiling point |
1800 °C, 2073 K, 3272 °F |
| Solubility in water | Insoluble |
| Solubility in acid | Soluble |
| Band gap | 2.137 eV |
| Structure | |
| Crystal structure | cubic |
| Hazards | |
| MSDS | SIRI.org |
| EU Index | 029-002-00-X |
| EU classification | Harmful (Xn) Dangerous for the environment (N) |
| R-phrases | R22, R50/53 |
| S-phrases | (S2), S22, S60, |
| NFPA 704 |
0
2
1
|
| Related compounds | |
| Other anions | Copper(I) sulfide Copper(II) sulfide Copper(I) selenide |
| Other cations | Copper(II) oxide Silver(I) oxide Nickel(II) oxide Zinc oxide |
| (verify) (what is: /?) Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Copper(I) oxide or cuprous oxide is the inorganic compound with the formula Cu2O. It is one of the principal oxides of copper. This red-coloured solid is a component of some antifouling paints. The compound can appear either yellow or red, depending on the size of the particles, but both forms degrade to copper(II) oxides in moist air.[1] Copper(I) oxide is found as the reddish mineral cuprite.
Contents |
Preparation
Copper(I) oxide may be produced by several methods.[2] Most straightforwardly, it arises via the oxidation of copper metal:
- 4 Cu + O2 → 2 Cu2O
Additives such as water and acids affect the rate of this process as well as the further oxidation to copper(II) oxides. It is also produced commercially by reduction of copper(II) solutions with sulfur dioxide. Aqueous cuprous chloride solutions react with base to give the same material. In all cases, the color is highly sensitive to the procedural details.
Formation of copper(I) oxide is the basis of the Fehling's test and Benedict's test for reducing sugars. These sugars reduce an alkaline solution of a copper(II) salt, giving a bright red precipitate of Cu2O.
It forms on silver-plated copper parts exposed to moisture when the silver layer is porous or damaged. This kind of corrosion is known as red plague.
Properties
The solid is diamagnetic. In terms of their coordination spheres, copper centres are 2-coordinated and the oxides are tetrahedral. The structure thus resembles in some sense the main polymorphs of SiO2, and both structures feature interpenetrated lattices.
Copper(I) oxide dissolves in concentrated ammonia solution to form the colourless complex [Cu(NH3)2]+, which easily oxidizes in air to the blue [Cu(NH3)4(H2O)2]2+. It dissolves in hydrochloric acid to give solutions of CuCl2-. Dilute sulfuric acid and nitric acid produce copper(II) sulfate and copper(II) nitrate, respectively.[3]
Structure
Cu2O crystallizes in a cubic structure with a lattice constant al=4.2696 Å. The Cu atoms arrange in a fcc sublattice, the O atoms in a bcc sublattice. The unit cell contains 4 Cu atoms and 2 O atoms. One sublattice is shifted by a quarter of the body diagonal. The space group is 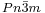 , which includes the point group with full octahedral symmetry.
, which includes the point group with full octahedral symmetry.
Semiconducting properties
In the history of semiconductor physics, Cu2O is one of the most studied materials, and many experimental observations and semiconductor applications have been demonstrated first in this material:
- Semiconductor
- Semiconductor diodes[4]
- Experimental demonstration of Wannier exciton series [5]
- Polariton propagation beats in a solid [5]
- Dynamical Stark effect of excitons [5]
- Phonoritons [6][7]
Today, it is still under investigation in semiconductor optics. Particularly people are trying to create a Bose-Einstein condensate of excitons in Cu2O.[5]
The lowest excitons in Cu2O are extremely long living and show a neV resonance, which is the narrowest bulk exciton resonance ever observed.[8] The associated quadrupole polaritons have low group velocity approaching the speed of sound. Thus, light moves almost as slowly as sound in this medium, which results in high polariton densities.
Another unusual feature of the ground state excitons is that all primary scattering mechanisms are known quantitatively.[9] Cu2O was the first substance where an entirely parameter-free model of absorption linewidth broadening by temperature could be established, allowing the corresponding absorption coefficient to be deduced. It can be shown using Cu2O that the Kramers–Kronig relations do not apply to polaritons.[5]
Applications
Cuprous oxide is commonly used as a pigment, a fungicide, and an antifouling agent for marine paints. Rectifier diodes based on this material have been used industrially as early as 1924, long before silicon became the standard.
See also
References
- ^ N. N. Greenwood, A. Earnshaw, Chemistry of the Elements, 2nd ed., Butterworth-Heinemann, Oxford, UK, 1997.
- ^ H. Wayne Richardson "Copper Compounds in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a07_567
- ^ D. Nicholls, Complexes and First-Row Transition Elements, Macmillan Press, London, 1973.
- ^ L. O. Grondahl, Unidirectional current carrying device, Patent, 1927
- ^ a b c d e C. F. Klingshirn, Semiconductor Optics, 3rd ed., Springer, 2006, Special:Booksources/354038345X
- ^ L. Hanke, D. Fröhlich, A.L. Ivanov, P.B. Littlewood, and H. Stolz "LA-Phonoritons in Cu2O" Phys. Rev. Lett. 83 (1999), 4365.
- ^ L. Brillouin: Wave Propagation and Group Velocity, Academic Press, New York, 1960.
- ^ J. Brandt, D. Fröhlich, C. Sandfort, M. Bayer, H. Stolz, and N. Naka, Ultranarrow absorption and two-phonon excitation spectroscopy of Cu2O paraexcitons in a high magnetic field, Phys. Rev. Lett. 99, 217403 (2007). doi:10.1103/PhysRevLett.99.217403
- ^ J.P. Wolfe and A. Mysyrowicz: Excitonic Matter, Scientific American 250 (1984), No. 3, 98.
External links
- National Pollutant Inventory: Copper and compounds fact sheet
- Chemical Land21 Product Information page
- Make a solar cell in your kitchen
- A Flat Panel Solar Battery
- Copper oxides project page
|
|||||